US reports record daily death toll from COVID-19 | Coronavirus pandemic News
The United States has reported a record number of deaths from COVID-19, as the worst outbreak in the world shows little sign of easing.
With a patient in California also confirmed to have the British variant of the virus, the country is also considering requiring COVID-19 tests from passengers from more countries as early as next week.
China has given conditional market approval to a vaccine being developed by state-owned Sinopharm.
Globally, nearly 82.7 million people have been diagnosed with the virus and 1,805,002 have died, according to data from Johns Hopkins University.
We are closing this blog now. These were the updates up to 05:00 GMT on Thursday, December 31:
Pakistan to buy 1.2 million doses of Sinopharm vaccine
Pakistan’s Minister of Science and Technology Fawad Chaudhry says the country will buy an initial 1.2 million doses of China’s Sinopharm vaccine.
It will be used to vaccinate front-line workers in the first quarter of 2021, he said in a post on Twitter.
China urges people to come forward for vaccination
A few more details from the press conference Chinese health officials held this morning, on its domestic vaccination plans.
It has been running an emergency use programme using at least three different shots since July, targetting those at highest risk.
Officials say the elderly and those with underlying conditions the next in line.
They were extremely keen to stress the Sinopharm vaccine’s safety, urging people to accept the inoculation and help China control the virus and achieve collective immunity.
“We call on people … to take an active part in vaccination to protect themselves, family members and others, which is also contributing to global epidemic control,” Zeng Yixin, an official with National Health Commission, told a briefing.
We’re live all day from #Wuhan one year on from the beginning of the outbreak which would go to infect millions are the world. Watch @AJEnglish for the latest on the approval of #China’s first #covid19 #vaccine & a study which is adding to doubts about official infection figures. pic.twitter.com/rQiInfBdtf
— Katrina Yu (@Katmyu) December 31, 2020
Countries in Asia-Pacific urge quieter New Year celebration
Many people are eager to see the back of 2020, but an increasing number of countries are urging people to celebrate at home for fear of sparking a surge in coronavirus cases.
Taiwan, which this month confirmed its first domestic case of COVID-19 since April, is urging people not to go out. The cities of Kaohsiung, Tainan, Taichung, Taoyuan, Chiayi and Keelung all said late on Wednesday they would cancel public attendance at events like fireworks displays.
It is a similar story in Australia, where Sydney – usually home to one of the world’s most spectacular fireworks displays – is trying to extinguish an outbreak that began in its northern beach suburbs. It recorded 10 new cases on Thursday.
New cases – three on Thursday and three the day before – have also emerged in Melbourne, prompting authorities there to order mandatory mask wearing in indoor venues from 5pm (06:00 GMT) on Thursday and limiting the number of people allowed in others’ homes.
Malaysia has also cancelled its year-end display around the Petronas Twin Towers amid a continued surge in cases. Kuala Lumpur and the surrounding state of Selangor have become the country’s new virus hotspots.
KLCC will not have the “Ambang 2021@KLCC” celebration due to CMCO, and no fireworks. However, the KLCC Lake Symphony and Plaza Fountain will operate till midnight 31 December 2020. Also, the Petronas Twin Towers will be lighted up till 2am. Thank you and regards, pic.twitter.com/WnKxS24jHx
— Corus hotel KL (@Corushotelkl) December 30, 2020
China officials questioned on Sinopharm data
Chinese officials have been asked about the data surrounding Sinopharm’s experimental vaccine.
They say the full data will be published “later” in medical journals in China and overseas.
Sinopharm’s China National Biotec Group says interim results from Phase III trials show the vaccine has an efficacy rate of 79.34 percent.
China gives conditional approval to Sinopharm vaccine
China says it has given conditional approval to the COVID-19 vaccine being developed by Sinopharm.
Officials speaking at a press conference in Beijing stressed China’s concern about safety and efficacy and said China was following international and WHO standards.
China hosts news conference on its vaccine
A press conference has just started in Beijing on China’s vaccine development progress.
We’ll bring you more details as they emerge.
China is working on a number of experimental vaccines, and is holding Phase III trials in nations including UAE, Brazil and Indonesia.
US reports record number of daily deaths
Johns Hopkins and the COVID Tracking Project say more than 3,900 people in the United States died from the coronavirus on December 30, a new daily record.
The Tracking Project, which is housed at The Atlantic, put the death toll at 3,903, while Johns Hopkins said it was even higher at 3,927.
Our daily update is published. States reported 1.6 million tests, 226k cases, a record 125,220 hospitalizations, and a record 3,903 COVID-19 deaths. Holiday reporting delays are still markedly affecting testing, case, and deaths figures. pic.twitter.com/HPV1cBbzLE
— The COVID Tracking Project (@COVID19Tracking) December 31, 2020
Fauci sees US gaining control of COVID-19 by autumn
Dr Anthony Fauci says he expects the US to achieve sufficient collective COVID-19 immunity through vaccinations to regain “some semblance of normality” by the autumn of 2021.
Fauci was speaking in an online discussion of the pandemic with California Governor Gavin Newsom, who says a highly infectious coronavirus variant originally found in Britain has been detected in his state, a day after the first known US case was documented in Colorado.
The United States’ top infectious diseases specialist said he was not surprised the variant had appeared.
“They make a living out of mutating. The more you mutate, the more you replicate,” Fauci said. Most, he added were “irrelevant”, but this “particular mutation does make the virus better at transmitting from one person to another.”
US considers extension of COVID-19 tests for travellers
The United States could expand coronavirus requirements for travellers arriving from more countries beyond the United Kingdom as early as next week, Reuters news agency is reporting, citing sources briefed on the matter.
The US Centers for Disease Control and Prevention (CDC) and other US agencies held a lengthy call with US airlines on Wednesday that discussed expanding the requirements, sources briefed on the call said.
You can read more on that story here.
 Military personnel hand out information to travellers arriving at JFK International airport in New York last week. The US is considering expanding a requirement for overseas passengers to have negative COVID-19 tests [Kena Betancur/AFP]
Military personnel hand out information to travellers arriving at JFK International airport in New York last week. The US is considering expanding a requirement for overseas passengers to have negative COVID-19 tests [Kena Betancur/AFP]
Hollywood productions shut down again as LA COVID-19 cases soar
Most Hollywood productions have shut down again until at least mid-January, the movie industry’s acting union announced, as COVID-19 cases soar to record levels in Los Angeles.
SAG-AFTRA said the majority of entertainment productions will “remain on hiatus until the second or third week of January if not later,” in a statement to members late Tuesday.
Canada to require negative test for people entering country
The Canadian government has said that passengers must have a negative COVID-19 test taken within three days before they arrive in the country.
Intergovernmental Affairs Minister Dominic LeBlanc said the measure will be implemented in the next few days.
Canada already requires those entering the country to self-isolate for 14 days and it has already banned all flights from the United Kingdom because of the new variant of COVID-19 spreading there.
 A man waits for a family friend while wearing a mask at Pearson airport arrivals, shortly after Toronto Public Health received notification of Canada”s first confirmed case of coronavirus, in Toronto, Ontario [File: Carlos Osorio/Reuters]
A man waits for a family friend while wearing a mask at Pearson airport arrivals, shortly after Toronto Public Health received notification of Canada”s first confirmed case of coronavirus, in Toronto, Ontario [File: Carlos Osorio/Reuters]
Irish government to consider additional COVID-19 measures – health minister
The Irish government will hold an unscheduled meeting on Wednesday to decide whether additional measures are required to control the “exponential growth” of COVID-19 in the country, Health Minister Stephen Donnelly said.
“We will look right across the spectrum… and see in light of the rising cases and the rise in hospital admissions what the appropriate thing to do is,” Donnelly told RTE radio.
The government has closed all bars and restaurants and will ban all household visits from January 1, but non-essential retail and schools remain open.
 A woman wearing a protective face mask walks past a man painting a mural, amid the coronavirus disease outbreak, in Dublin, Ireland [File: Clodagh Kilcoyne/Reuters]
A woman wearing a protective face mask walks past a man painting a mural, amid the coronavirus disease outbreak, in Dublin, Ireland [File: Clodagh Kilcoyne/Reuters]
New strain spreading faster than all forecasts – Irish PM
A new strain of COVID-19 that reached Ireland from the United Kingdom is spreading faster than the country’s most pessimistic forecasts, Prime Minister Micheal Martin said.
“While international research for this new variant is ongoing, it is already very clear that we are dealing with a strain of the disease that spreads much, much more quickly,” Martin said in a televised address announcing a tightening of public-health restrictions for the next four weeks.
Argentine regulator approves AstraZeneca vaccine
Argentina’s regulator approved the COVID-19 vaccine developed by AstraZeneca and Oxford University for emergency use in the country, AstraZeneca said in a statement.
AstraZeneca said the approval by the National Administrator for Food and Medical Technology (ANMAT) approval made Argentina “one of the first countries in the world to authorise” the drug, after the UK regulator gave the green light for its widespread roll-out.
 Daniela Zapata, 42, receives an injection with the Sputnik V (Gam-COVID-Vac) vaccine against the coronavirus disease (COVID-19) at Dr Pedro Fiorito hospital in Avellaneda, on the outskirts of Buenos Aires, Argentina, December 29, 2020 [Agustin Marcarian/Reuters]
Daniela Zapata, 42, receives an injection with the Sputnik V (Gam-COVID-Vac) vaccine against the coronavirus disease (COVID-19) at Dr Pedro Fiorito hospital in Avellaneda, on the outskirts of Buenos Aires, Argentina, December 29, 2020 [Agustin Marcarian/Reuters]
French government sees no immediate need for lockdown – spokesman
France is not envisaging local lockdown measures to contain the COVID-19 pandemic based on the current pace at which the disease is spreading, government spokesman Gabriel Attal said.
“The rate at which the virus is circulating does not justify bringing in local lockdown measures,” Attal told BFM TV.
Attal said there was no shortage of vaccine doses in France and said the country would meet its goal of vaccinating a million people by February, after growing criticism over its slow start in administering doses compared with other European nations.
Qatar’s emir receives vaccine shot
Qatar’s Emir Sheikh Tamim bin Hamad Al Thani announced on social media that he had received the coronavirus vaccine.
“Today I took the COVID-19 vaccine, and I wish everyone safety and protection from this epidemic,” the emir said on his Instagram account.
US likely to approve AstraZeneca vaccine in April
The United States is expected to approve the low-cost UK-backed AstraZeneca/Oxford vaccine in April, a senior official said, more than three months after Britain’s green light on Monday.
Moncef Slaoui, the chief adviser to Operation Warp Speed, the military-led US effort for vaccines, did not question Britain’s move but told reporters that US trials would be complete for approval “sometime in early April.”
England delays return of schools as COVID-19 cases surge
Secondary school children in England will return to the classroom later than planned to enable the roll out of mass COVID-19 testing, Education Minister Gavin Williamson said.
Pupils in exam years will return from Jan 11 and all secondary and college students will return full time on January 18.
“This will break those chains of transmission that are making infection rates shoot up, this in turn will make it safer for more children to physically return to school,” he told legislators.
 The UK has more than 2.1 million cases and more than 50,000 deaths [File: Christopher Furlong/Getty Images]
The UK has more than 2.1 million cases and more than 50,000 deaths [File: Christopher Furlong/Getty Images]Amid virus fears, China urges workers to skip holiday travel
China is encouraging tens of millions of migrant workers not to travel home during next February’s Lunar New Year holiday to prevent further spread of the coronavirus, disrupting its most important time for family gatherings.
The measure from the National Health Commission is not a direct travel ban but is still extraordinary because the traditional holiday is the only time of the year when many workers have the opportunity to travel home to see their families.
Turkey reports more than 15,600 new COVID-19 infections
Turkey has reported 15,692 more infections and 254 new fatalities from coronavirus over the past 24 hours.
According to Health Ministry data, the new cases include 2,612 symptomatic patients. The tally of infections exceeded 2.19 million, while the death toll climbed to 20,642.
Tottenham, Fulham postponed over virus tests: Premier League
Tottenham’s Premier League home match against Fulham was postponed less than three hours before the scheduled kick-off on after a coronavirus outbreak at the visiting club.
Fulham returned a number of positive test results on Tuesday and, after more came in on Wednesday, the Premier League decided to call the match off. Kick-off was due to be at 18:00 GMT.
It is the third top-flight game to be wiped out by the virus, following Newcastle’s match with Aston Villa earlier this month and Monday’s game between Everton and Manchester City.
We can confirm that our Premier League home fixture against Fulham, scheduled to take place this evening, has been postponed.#THFC ⚪️ #COYS
— Tottenham Hotspur (@SpursOfficial) December 30, 2020
Germany set for longer lockdown as death figures spike
German officials said they won’t be able to relax lockdown restrictions in early January as the country recorded more than 1,000 deaths in one day for the first time.
That figure was likely swollen by delayed reporting but underlined the severity of the situation.
Germany, the European Union’s most populous country, shut restaurants, bars, sports and leisure facilities on November 2.
 A poster demands “When it gets tight – mask on!”, as the spread of the coronavirus disease (COVID-19) continues, at a pedestrian zone in Konstanz, Germany December 15, 2020 [File: Arnd Wiegmann/Reuters]
A poster demands “When it gets tight – mask on!”, as the spread of the coronavirus disease (COVID-19) continues, at a pedestrian zone in Konstanz, Germany December 15, 2020 [File: Arnd Wiegmann/Reuters]AstraZeneca says working to get its vaccine approved in Brazil
AstraZeneca said it is working efficiently and transparently to bring its vaccine to Brazil as fast as possible, adding that it remains committed to seeking full regulatory approval in Brazil after authorisation in the UK.
In a statement, AstraZeneca said it would keep up the ongoing submission of its late-stage trial results but made no mention of seeking emergency use approval – a process that Pfizer has described as cumbersome in Brazil.
Zimbabwe delays new school term due to rising COVID-19 cases, cyclone
Zimbabwe has postponed the re-opening of schools planned for next week, the government said, due to a surge in coronavirus infections and a tropical storm sweeping through the region.
The government had set January 4 as the date to reopen primary and secondary schools, after many students missed class for much of last year as the country tried to curb the spread of COVID-19.
Zimbabwe has recorded more than 13,000 cases of COVID-19 and 359 deaths.
 Daily cases have averaged more than 100 this week, compared with less than 50 last month [File: Philimon Bulawayo/Reuters]
Daily cases have averaged more than 100 this week, compared with less than 50 last month [File: Philimon Bulawayo/Reuters]
WHO urges fair vaccine distribution to all
The head of the World Health Organization, marking a year since the first cases of the novel coronavirus were reported by China, urged countries to ensure that vaccines are made available to people at risk everywhere, not just in rich nations.
Tedros Adhanom Ghebreyesus, WHO director-general, appealed for $4bn to buy COVID-19 vaccines for distribution in lower and middle-income countries through the COVAX vaccine facility.
“This is the challenge we must rise to in the New Year,” Tedros said in a video message issued a day before the first anniversary of China reporting the first cases of pneumonia of unknown origin to the UN health agency.
Ukraine signs up for China’s Sinovac vaccine
Ukraine has signed contract to buy 1.8 million doses of China’s Sinovac COVID-19 vaccine, the presidential office said, with the shots expected in “the shortest possible time”.
“We conducted very active, multi-month negotiations and got a concrete result,” President Volodymyr Zelenskiy was quoted by his office as saying.
Prime Minister Denys Shmygal said this month the first shots could arrive in February.
Israel records more than 5,500 cases in one day
Israel’s health ministry reported the highest number of new coronavirus infections in over two months.
The ministry recorded 5,585 new cases from the previous day, which is the highest daily rate since the beginning of October, when Israel was in the midst of a national lockdown.
Hello. This is Usaid Siddiqui in Toronto, Canada taking over from my colleague Virginia Pietromarchi.
Portugal PM resumes public activities
Portugal’s Prime Minister Antonio Costa has tested negative for COVID-19 and will resume in-person public appearances from today, his cabinet said in a statement.
Costa had spent two weeks in self-isolation following a meeting with French President Emmanuel Macron, who had contracted the disease.
 French President Emmanuel Macron and Portugal’s Prime Minister Antonio Costa wave to journalists as they enter the Elysee Palace in Paris, France [File: Gonzalo Fuentes/Reuters]
French President Emmanuel Macron and Portugal’s Prime Minister Antonio Costa wave to journalists as they enter the Elysee Palace in Paris, France [File: Gonzalo Fuentes/Reuters]
China urges workers to skip holiday travel
China is encouraging tens of millions of migrant workers not to travel home during February’s Lunar New Year holiday to prevent further spread of the coronavirus.
The measure from the National Health Commission (NHC) is not a direct travel ban but is still extraordinary because Lunar New Year is China’s most important traditional holiday and the only time of the year when many workers have the opportunity to travel home to see their families.
The NHC said it was encouraging provincial governments to persuade workers to follow the suggestion while taking into account their personal wishes. It also said workers who stay behind should be paid overtime and offered other opportunities to take a vacation.
Swiss gov’t sticks to current restrictions
The Swiss government decided against imposing further restrictions to prevent the spread of the coronavirus amid faster-spreading variants which recently entered the country.
“The Federal Council has conducted a detailed analysis of the current epidemiological situation. This remains worrying due to the high level of infection and the appearance of two new virus variants in Switzerland,” the government said in a statement.
“However, the Federal Council has come to the conclusion that the measures taken on December 18…are appropriate and do not need to be tightened.”
 Skiers pass the restriction signs to line up at the chairlift at Les Portes du Soleil ski resort during the global outbreak of the coronavirus disease in Les Crosets, Switzerland [Denis Balibouse/Reuters]
Skiers pass the restriction signs to line up at the chairlift at Les Portes du Soleil ski resort during the global outbreak of the coronavirus disease in Les Crosets, Switzerland [Denis Balibouse/Reuters]
Cases surge in Latin America amid vaccine roll out
Germany expects quick EU approval of AstraZeneca/Oxford shot
Germany expects the European Union to give quick approval to the coronavirus vaccine developed by Oxford University and AstraZeneca, its top vaccines official said.
Klaus Cichutek, head of the Paul Ehrlich Institute, said that, thanks to the rolling EU review of the AstraZeneca vaccine’s effectiveness, it would be possible to take a quick decision once a formal application was submitted.
No such application had been received by the European Medicines Agency (EMA) by Wednesday morning, Cichutek told reporters, but a debate in its councils would follow as soon as it arrived.
Expert welcomes vaccine approval
Jeff Lazarus, head of the Health System research group at the Institute for Global Health in Barcelona, spoke to Al Jazeera over the AstraZeneca/Oxford vaccine following its approval by UK regulators. Here are some of his thoughts.
“This is fantastic news. The issue of storage is vital because many countries simply don’t have the refrigeration possibilities that we have in higher-income countries. And the price is lower which will increase access and allow for scaling up much faster,” he said.
The AstraZeneca/Oxford shot can be kept at a normal fridge temperature, making it easier to store and distribute compared with the Pfizer/BioNTech vaccine which requires deep-freezing equipment.
“Viruses mutate, we have already seen it … we have the new variant identified and there is likely to be further mutations,” he added, referring to the new COVID-19 strain first identified in the UK which has now been detected in other countries.
“So we need to get as many people vaccinated as quickly as possible. And at the same time, which is going to be one of the main challenges, we need to continue carrying the pandemic measures: face masks, hand washing, no large gatherings to avoid other transmissions.”
Cases in Wuhan may be 10 times higher than reported: study
The number of cases in the Chinese city where the pathogen was first detected may have been 10 times higher than official figures suggest, according to a study by health authorities in Wuhan.
About 4.4 percent of the city’s 11 million residents had developed antibodies against the novel virus by April, read the report by the Chinese Centre for Disease Control (CDC).
That correlates to approximately 480,000 infections in Wuhan by April, nearly 10 times the official tally to date of 50,000 cases in the city.
The discrepancy revealed by the CDC’s data may “point to potential underreporting due to the chaos in late January and early February, when a large number of people were not tested or were not tested accurately for COVID-19,” Huang Yanzhong, a senior fellow for global health at the Council on Foreign Relations, told AFP news agency.
India races to find UK arrivals to halt new virus strain
Indian authorities were trying to track down tens of thousands of people who entered the country from Britain in recent weeks as cases of a new and fast-spreading coronavirus strain more than doubled in 24 hours.
They have launched efforts to locate about 33,000 people who flew to India in the last month from the UK after 20 people tested positive for the new, more virulent strain, up by 14 cases since Tuesday.
 India launched efforts to locate approximately 33,000 people who flew to India in the last month from the UK after 20 people tested positive for the new strain [Francis Mascarenhas/Reuters]
India launched efforts to locate approximately 33,000 people who flew to India in the last month from the UK after 20 people tested positive for the new strain [Francis Mascarenhas/Reuters]
India also extended the ban on flights to and from Britain by a week to January 7.
“Comprehensive contact tracing has been initiated for co-travellers, family contacts and others,” the Indian health ministry said Tuesday, referring to those who flew between November 25 and December 23, when the government suspended air links with Britain.
It remains unclear how many arrivals from Britain it has traced so far.
Vaccines’ costs and differences
Here is a quick look at some of the differences between leading vaccines:
The Pfizer/BioNTech vaccine was found to be 95 percent effective with no serious side effects. It costs about $20 per shot and it requires two doses each person. It needs to be transported and stored in deep-freeze equipment.
The Moderna shot had 94 percent success in trials. It can be stored in a regular freezer, but it is more expensive as it costs approximately $35 per shot and it requires two doses.
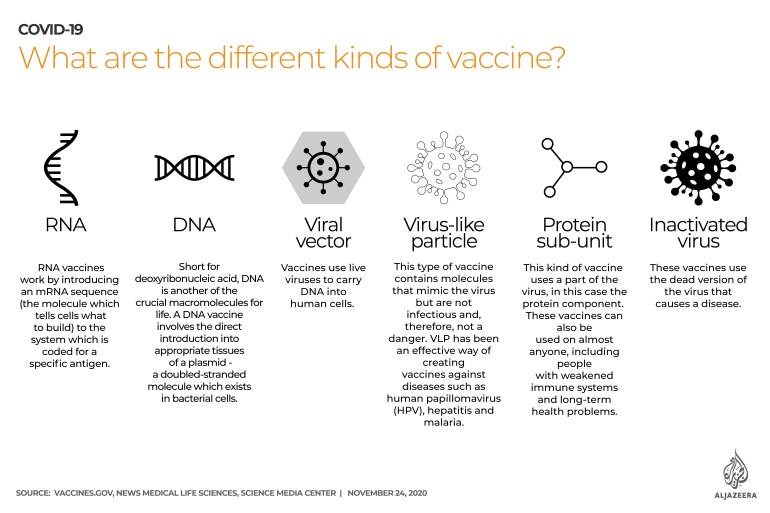
The AstraZeneca/Oxford University vaccine is on average 70 percent effective after one injection, but it should pass 90 percent after a second one. It can be stored in a normal freeze and it cost $3 per dose.
Russia’s Sputnik V costs less than $10 per shot, but it raised criticism as people were given the first dose before mass human trials. Health officials claim it is 91 percent effective after two shots.
AstraZeneca/Oxford shot should be effective for new strain: CEO
The newly approved AstraZeneca-Oxford COVID-19 vaccine should be effective against a rapidly spreading new variant of the disease.
“Our belief at this point is that this vaccine should be effective against the variant,” AstraZeneca’s Chief Executive Pascal Soriot told BBC radio.
Lockdown in England to be widened: Health Minister
As the Oxford/AstraZeneca vaccine was approved, UK Health Secretary Matt Hancock said that lockdown measures will be extended to curb the rapid growth of COVID-19 cases.
In an interview with BBC television, the minister also said that hundreds of thousands of doses of the shot are ready to be rolled out in the country starting from Monday.
Taiwan secures AstraZeneca vaccine
Taiwan has agreed to buy almost 20 million doses of the vaccine, including 10 million from AstraZeneca Plc, just before the drug company shot was approved by UK regulators.
The news came as the government confirmed to have detected the first case of the new COVID-19 variant on the island.
Taiwan has kept the pandemic at bay thanks to early and effective prevention and strict quarantine of all arrivals, with imported cases accounting for almost all of its tally of 798 cases, including seven deaths.
The island’s Central Epidemic Command Centre said apart from AstraZeneca, it had agreed to buy 4.76 million doses from global vaccine programme COVAX, and was still in talks with another company it did not name.
 Taiwan has kept the pandemic at bay thanks to early and effective prevention and strict quarantine of all arrivals [Ann Wang/Reuters]
Taiwan has kept the pandemic at bay thanks to early and effective prevention and strict quarantine of all arrivals [Ann Wang/Reuters]
Oxford/AstraZeneca shot: ‘Good news, but might not be enough’
Al Jazeera’s Rory Challands comments on the news over the authorisation of the Oxford-AstraZeneca vaccine.
“It’s a huge step forward and it could not have come at a better time because the situation in the UK has been worsening significantly in the last few days,” Challands said reporting from London.
For two days running, a record number of people got infected in the UK due to a new faster-spreading COVID-19 strain. In the past 24 hours, more than 53,300 new cases were registered, while more than 41,300 new people tested positive the previous day.
The authorisation is also good news “because this injection is much easier to use, to transport and administer than the Pfizer/BioNTech which is the one that has been used until now,” said Challands.
However, the new COVID-19 variant has been spreading so widely and fast, Challands explained, that some medical experts are warning 2021 could be worse than 2020 in terms of hospitalisations and deaths.
“So there is a high likelihood that even with the vaccine being authorised for immediate roll out, there will need to be tougher measures taken when it comes to people’s life’” he added.
Oxford/AstraZeneca vaccine: High-risk group to be prioritised
Starting today the National Health Service “will prioritise giving the first dose of the vaccine to those in the most high-risk groups,” read a statement from the Department of Health and Social Care.
“With two vaccines now approved, we will be able to vaccinate a greater number of people who are at highest risk, protecting them from the disease and reducing mortality and hospitalization,” it added.
According to the statement, the Joint Committee on Vaccination and Immunisation (JCVI) “has advised the priority should be to give as many people in at-risk groups their first dose, rather than providing the required two doses in as short a time as possible.”
It added: “Everyone will still receive their second dose and this will be within 12 weeks of their first. The second dose completes the course and is important for longer-term protection.”
‘A moment of hope’
UK Health Secretary Matt Hancock welcomed the news over the authorisation of the Oxford/AstraZeneca jab which paves the wave for mass vaccination.
“Brilliant to end 2020 with such a moment of hope,” Hancock said on Twitter. “The coronavirus vaccine is our way out of the pandemic – now we need to hold our nerve while we get through this together,” he added.
Brilliant to end 2020 with such a moment of hope: the @UniofOxford / @AstraZeneca #coronavirus vaccine has today been authorised for use by @mhragovuk
The #coronavirus vaccine is our way out of the pandemic – now we need to hold our nerve while we get through this together.
— Matt Hancock (@MattHancock) December 30, 2020
The “truly fantastic news” was greeted by Prime Minister Boris Johnson as well who said it was a “triumph for British science”.
“We will now move to vaccinate as many people as quickly as possible,” he added on Twitter.
It is truly fantastic news – and a triumph for British science – that the @UniofOxford /@AstraZeneca vaccine has been approved for use.
We will now move to vaccinate as many people as quickly as possible. pic.twitter.com/cR4pRdZJlT
— Boris Johnson (@BorisJohnson) December 30, 2020
AstraZeneca-Oxford COVID-19 vaccine approved for use in UK
A coronavirus vaccine developed by drug firm AstraZeneca and Oxford University has been approved for use in Britain, the government announced on Wednesday, paving the way for a mass roll-out.
A government spokesman said it has accepted a recommendation from the Medicines and Healthcare products Regulatory Agency (MHRA) “to authorise Oxford University/AstraZeneca’s COVID-19 vaccine for use”, making Britain the first nation to approve the jab.
AstraZeneca Chief Executive Pascal Soriot said “Today is an important day for millions of people in the UK who will get access to this new vaccine. It has been shown to be effective, well-tolerated, simple to administer and is supplied by AstraZeneca at no profit”.
Abu Dhabi approves remote learning for two weeks
Abu Dhabi has approved remote learning for schools in the United Arab Emirates for the first two weeks of the new term starting on January 3 to protect the health of students and staff, the Abu Dhabi media office said on Twitter.
The Abu Dhabi Emergency, Crisis and Disasters Committee, in coordination with Abu Dhabi Department of Education and Knowledge, has approved remote learning for the first two weeks of the new school term, starting 3 January 2021. pic.twitter.com/1dk8nPTHv8
— مكتب أبوظبي الإعلامي (@admediaoffice) December 30, 2020
China Sinopharm vaccine found to be 79 percent effective
Chinese pharmaceutical giant Sinopharm said Phase III trials of its experimental vaccine have found the shot to be 79 percent effective against COVID-19.
“Sinopharm CNBG Beijing’s inactivated coronavirus vaccine exhibits safety after vaccination … the protective effect of the vaccine against COVID-19 is 79.34%,” the Beijing Institute of Biological Products – a Sinopharm subsidiary – said in a statement.
Possible ‘explosion’ of cases in Tokyo: governor
The coronavirus situation in Tokyo is quite severe and the Japanese capital could potentially face an “explosion” of infections, Tokyo Governor Yuriko Koike warned ahead of the New Year holiday.
“Please emphasise life over fun,” she told a news conference, calling on people to stay at home as much as possible over the holiday, one of Japan’s longest, in which people hold parties, gather in their homes and return to their hometowns from the capital.
 A man sits next to a new year’s decoration in front of a temple in Tokyo, Japan [Kim Kyung-Hoon/Reuters]
A man sits next to a new year’s decoration in front of a temple in Tokyo, Japan [Kim Kyung-Hoon/Reuters]
US detects first case of new variant in Colorado
The first reported US case of the COVID-19 variant that has been seen in the United Kingdom has been discovered in Colorado, Governor Jared Polis announced Tuesday.
The variant was found in a man in his 20s who is in isolation southeast of Denver and has not travelled around recently, state health officials said.
The Colorado State Laboratory confirmed the virus variant, and the Centers for Disease Control and Prevention was notified.
Read the full story here.
